Fitbit and Dexcom!
Fitbit and Dexcom to Develop Continuous Glucose Monitoring (CGM) Experience for People Living with Diabetes
press release
SAN FRANCISCO & SAN DIEGO--(BUSINESS WIRE)-- Fitbit (NYSE:FIT), the leading global wearables brand, and DexCom, Inc. (NASDAQ:DXCM), the leader in continuous glucose monitoring (CGM) for people with diabetes, today announced a collaboration to develop and market products to help people better manage their diabetes and get a more complete picture of their overall health with easy-to-use mobile tools.
This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20170907005577/en/
The first planned initiative is to bring Dexcom CGM data to Fitbit's new smartwatch, Fitbit Ionic. Through this experience, Dexcom CGM users on either Android or iOS devices would be able to see both activity and glucose levels, right on their wrist.
"The strength of our brand and our ability to track critical health metrics continuously for up to 4+ days1, coupled with Dexcom's market leadership in CGM, present a powerful combination that we hope will help millions of people better manage their diabetes," said James Park, CEO of Fitbit. "With Ionic, we are focused on driving positive health outcomes and more health focused tools, and this collaboration is a wonderful example of how we plan to bring that vision to our users."
Dexcom CGM Display on Fitbit Ionic will provide data for those living with diabetes
The World Health Organization estimates2 that more than 400 million people around the world are living with diabetes. For those individuals being able to see both physical activity and glucose can be a vital tool for effectively managing their diabetes.
"The collaboration between Dexcom and Fitbit is an important step in providing useful information to people with diabetes that is both convenient and discreet," said Kevin Sayer, President and CEO, Dexcom. "We believe that providing Dexcom CGM data on Fitbit Ionic, and making that experience available to users of both Android and iOS devices, will have a positive impact on the way people manage their diabetes."
A health and fitness first platform, Ionic offers a highly personalized experience not previously seen in other smartwatches. Ionic features a relative SpO2 sensor, industry-leading GPS tracking, on-device dynamic workouts, improved heart rate tracking, and water resistance up to 50 meters. Plus, smart features like contactless payments, on-board music, smart notifications, and a variety of popular apps and clock faces available in the Fitbit App Gallery. Ionic also has all the core features from Fitbit like 4+ day battery life, automatic activity and sleep tracking, and cross-platform compatibility.
In addition to the Dexcom CGM display for Fitbit Ionic, with Fitbit's in-app Community, Dexcom CGM users will now be able to connect with millions of people, where they can ask questions, seek support and share successes in managing their health.
The companies are targeting availability as soon as possible in 2018 and will continue to explore opportunities to work together to develop tools and resources aimed at helping people better manage their diabetes.
Dexcom is a sponsor of the Juicebox Podcast. You can use this link to find out more about our favorite continuous glucose monitoring system.
Decision Moves Continuous Glucose Monitors One Step Closer to Medicare Coverage
great news from JDRF.org !!
January 12, 2017
Continuous glucose monitoring (CGM) devices approved by the FDA for use in making diabetes treatment decisions are durable medical equipment, according to a decision today by the Centers for Medicare & Medicaid Services (CMS). That determination removed a major roadblock to the devices’ coverage under Medicare. Today’s decisions mean that CGMs approved by the FDA for use in making diabetes treatment decisions are eligible for reimbursement under Medicare.
Today’s decision creates a pathway for Medicare coverage for the devices that will bring the nation’s largest insurer in line with the vast majority of the country’s private payers. Although the significant benefits of CGM use have been known since 2008, CMS had previously refused to consider covering the devices under Medicare, saying they did not meet the statutory definitions of durable medical equipment or any other category the agency could cover. Today’s decision removes that impediment.
“JDRF is encouraged by this decision, which will bring us closer to Medicare coverage for continuous glucose monitors,” said Aaron J. Kowalski, PhD, JDRF’s Chief Mission Officer. “I want to thank the tireless JDRF advocates and Congressional champions who have made this progress possible.”
the entire press release can be found here on the JDRF website
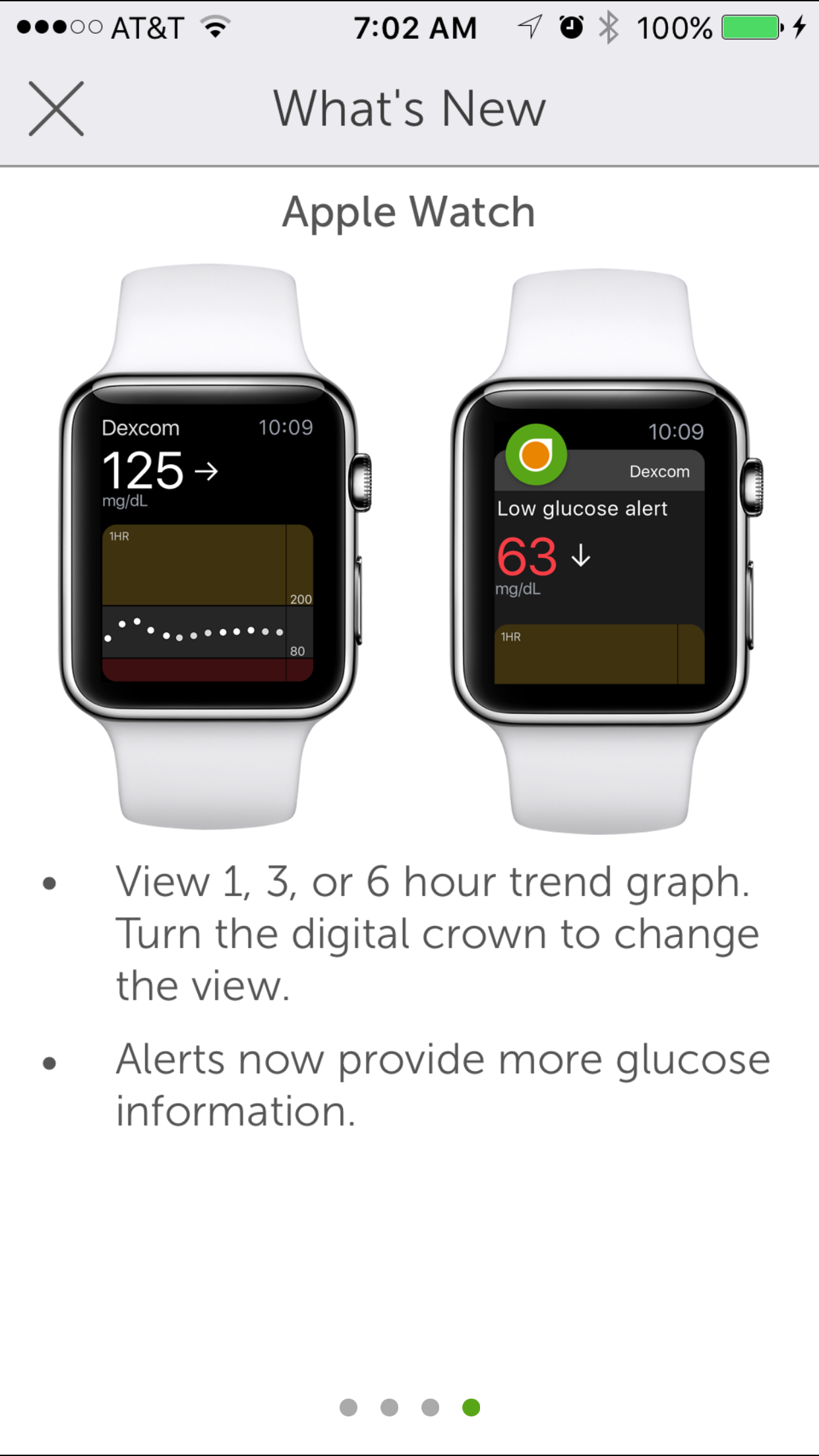
Dexcom G5 Mobile Gains Apple Watch Complications!
Do you know what watch complications are?
Complications are the newest feature for the Apple Watch app. Dexcom G5 users with an Apple Watch can choose from 4 different watch faces and by just lifting their wrist they can quickly and easily see their glucose level and trend arrow.
“Complications are small visual elements on the watch face that communicate important information to the user. The term complication comes from watch making, where the addition of features added complexity to the watch construction. Complications are visible whenever the user looks at the watch face, and users can customize which complications are displayed. The number of slots available for complications on a given watch face varies, but most support at least two or three complications.”
Dexcom representatives told me....
click to expand
"We have seen many social media posts indicating that our users have purchased the Apple Watch primarily because Dexcom CGM glucose data is available on the watch. We are excited that this release will allow our users with an Apple Watch even greater convenience for those who want this important information in an easy-to-use and discreet form."
click to expand
"Close collaboration took place with Apple to get the watch platform to support our use case of 288 updates/day to make this feature possible for CGM. The Dexcom Watch Face was shown at last year’s World Wide Developers Conference in September, and its currently being promoted on Apple’s website in two locations, (1) on great new features of watchOS3, and (2) third-party ecosystems that make the Apple Watch great. Below are screenshots from both of Apple’s website locations."
click to expand
"As part of Dexcom’s commitment of continuous improvement in the quality and user experience of our apps, the 1.6 release also includes several sustaining improvements and enhancements."
Dexcom apps are available for iOS here.
Click here to learn more about Dexcom
Dexcom G5 App v1.6 and Apple Watch running watchOS 3 or later required. US only.
FDA: No Fingerstick Needed with Dexcom G5 CGM
by John Gever
Managing Editor, MedPage TodayDecember 20, 2016
WASHINGTON -- The FDA has approved an expanded indication for Dexcom's G5 Mobile continuous glucose monitor, allowing it to be used alone without supplementary fingerstick glucose testing.
"This is the first FDA-approved continuous glucose monitoring system that can be used to make diabetes treatment decisions without confirmation with a traditional fingerstick test," the agency said in announcing the decision. Previously, the system had been approved as an adjunct to fingerstick testing.
But the new approval does not mean patients can forget about fingerstick testing entirely. "Users are warned that the system must be calibrated using a fingerstick blood sample at least once every 12 hours," the FDA stressed.
In deciding to expand the indication, the FDA relied on findings from two 7-day clinical trials involving a total of 130 adults and children as young as 2. Results from the continuous monitor were compared with those from standard fingerstick meters and from lab tests of blood glucose. Those studies indicated that the continuous monitor was accurate enough to be used alone to guide treatment.
The FDA noted that the device still comes with risks including hyper- and hypoglycemia stemming from inaccurate readings. Also, redness and irritation may occur at the site where the device's skin patch is applied.
The agency also indicated that acetaminophen-containing products can "falsely raise glucose readings" from the device.
App Update: Dexcom G5 Mobile
Available today: Dexcom G5 Mobile Update: v1.5.1
I'm excited for the big update that is in the works for the future, but for now... this is a nice little improvement to the app.